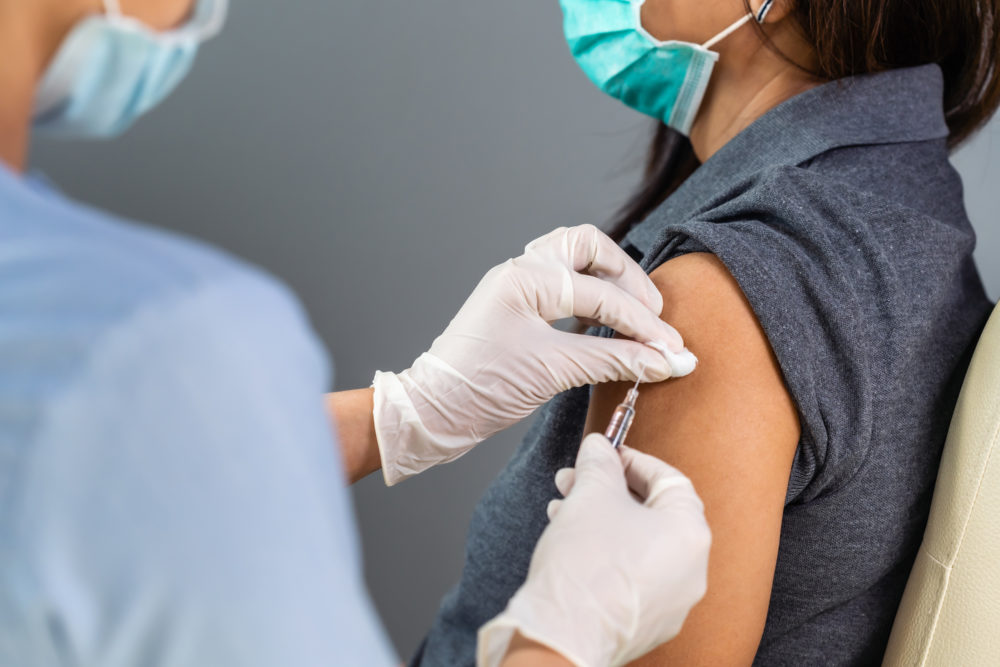
When my 101-year-old patient told me she planned to pass on getting a COVID-19 vaccine, I realized there was a problem. People who are living in the assisted living facilities are among the first to be given this opportunity, and she declined it. In the days that followed, I realized more patients were hesitant to get vaccinated. Here are answers to the questions I’m frequently asked. As for me, when I had the opportunity to get vaccinated, I took it.
What is herd immunity?
This is when a majority of the community has established immunity by vaccination (or through infection) where transmission of the virus is less likely. Herd immunity protects the vulnerable who can’t be vaccinated such as children under 16, pregnant women and those allergic to certain components of the vaccine. If you’re pregnant, discuss your options with your obstetrician. Currently, the vaccine is not approved for children younger than age 16. The Pfizer vaccine is for children older than age 16, and Moderna is for young adults age 18 and older. As adults get vaccinated, they protect children from getting the virus until they are old enough to get it.
What does emergency use authorization mean?
Emergency use authorization—also known as EUA—is an authority given to U.S. Food and Drug Administration to help fight a public health emergency such as this pandemic. It allows the FDA to make a treatment or vaccine available, if there’s strong evidence it will benefit patients.
Was EUA granted for the COVID-19 vaccine?
The FDA established a minimum criteria that must be met for its approval for EUA. The vaccine had to be at least 50 percent effective in reducing the coronavirus infection and a safety monitoring of at least eight weeks of data available prior to approval. Both Pfizer and Moderna vaccines have shown to be over 94 percent effective in reducing the corona virus infection with no major side effects. It’s also important to know that of those vaccinated, and who still contracted the COVID virus infection, none experienced severe symptoms of the disease, meaning they didn’t required ICU-level care.
Does an EUA vaccine means it’s less safe?
The emergency use was approved because there was more benefit from receiving the vaccine than risk from getting it. Two independent committees reviewed the trial data and made recommendations to the FDA for its approval. No steps were skipped in the process of developing the vaccines.
How was the vaccine developed so rapidly?
This is the first time mRNA technology was used for a vaccine, but it has been used in cancer treatment before. This vaccine is a testament to great things that can be accomplished if private companies and government collaborate on a project. Using a technology that already existed and having the federal government absorb the risk, allowed the rapid development and availability of the vaccines. Additionally, there was a ready pool of volunteers for the trials. The FDA usually requires vaccine trials to include about 3,000 people. Pfizer vaccine trials included 40,000 people; Moderna drug trials included 30,000 people.
What are the side effects of the vaccine?
It’s common to get a slight fever, headache, chills, or body ache after the vaccine, though it’s usually short-lived. (Personally, 12 hours after getting the vaccine, my arm was very sore. I feel assured that my body is working on my immunity to the virus.)
How long do I have immunity?
If you had COVID-19, medical scientists believe you have natural immunity for 90 days, but there are studies that claim you could have natural immunity for five to seven months after the infection. If you’re vaccinated, it’s unclear how long you have immunity, and will depend on the virus’s ability to mutate. Experts believe it could take a long time before the virus can effectively mutate to make the vaccine ineffective. It’s possible you might need a COVID vaccine annually, much like the flu vaccine.
If I get the vaccine, how long before I have immunity?
Both Pfizer and Moderna vaccines require two doses. Pfizer is three weeks apart and Moderna is four weeks apart. It’s expected that it will be most effective about one to two weeks after a second dose. It is possible that you can get infected with the coronavirus during this vaccination period.
What if I had COVID?
The recommendation is to get the vaccine, 90 days after the onset of the infection. The vaccine is expected to improve and increase the duration of your immunity.
Final thoughts
The long-term side effects of the COVID vaccine are unknown at this time. However, the alternative to not being vaccinated is to remain isolated, masked and hope enough people get the vaccine so we develop herd immunity. My advice? It’s critical that we each do our part to protect ourselves, family, coworkers and community. As a mother, I hope every adult is vaccinated to protect the children. As a physician, I know this to be the most effective way of putting an end to the pandemic.
Author
-

Rajina Ranadive, M.D., is a board certified internal medicine physician with the St. Joseph’s Medical Group. She is also the medical director of the Petaluma Post-Acute Rehab. She can be reached at (707) 763-0802.
View all posts



